3D cell culture of the liver
Papers selected and presented by PhD student Jonathan Temple (he will officially become Editor once he has published his first article).
Editor Raphaël Lévy
Jessica H. Brown, Prativa Das, Michael D. DiVito, David Ivancic, Lay Poh Tan, Jason A. Wertheim
PubMed: 29454157 DOI: 10.1016/j.actbio.2018.02.009
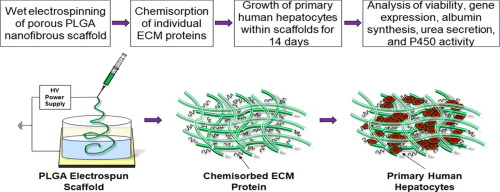
Brown et al. developed a three-dimensional (3D) nanofibrous scaffold made from poly(L-lactide-co-glycolide) (PLGA) polymer using a newly optimized wet electrospinning technique. The resulting scaffold had a highly porous structure when compared with conventional ‘dry’ electro-spun PLGA scaffolds (Figure 1).
Figure 1. Fabrication process of wet electro-spun PLGA scaffold with chemisorbed ECM proteins; collagen and fibronectin (reproduced from Brown et al's grahical abstract).
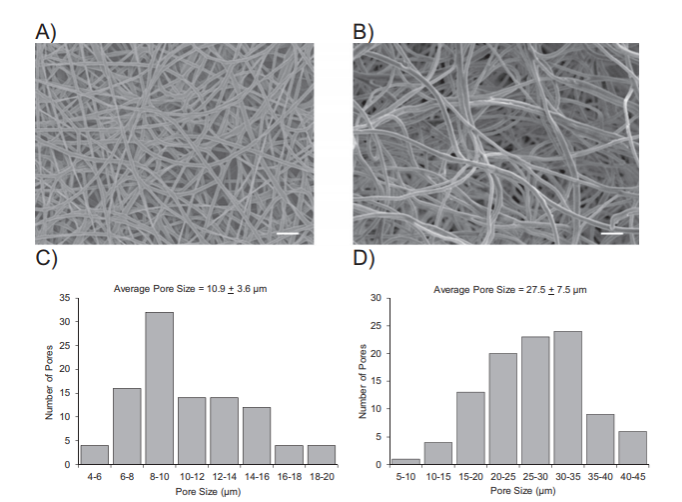
Extracellular matrix (ECM) proteins (type I collagen or fibronectin) of varying concentrations were chemically linked to electro-spun PLGA using amine coupling. Cell-laden nanofiber scaffolds were tested for albumin and urea secretion along with cytochrome P450 expression and activity. Brown et al. used SEM to compare electro-spun scaffold collected from the conventional flat platform with a smaller pore size compared to scaffolds developed using wet electrospinning (Figure 2). They discuss how the pore size in the wet scaffold better mimics the acellular architecture of the human liver whilst also improving the penetration of the scaffold by cells, with most cells on the dry scaffold located on the surface.
Figure 2. SEM micrograph and pore size values for both dry and wet electro-spun PLGA scaffold (reproduced from Brown et al's Figure 1)
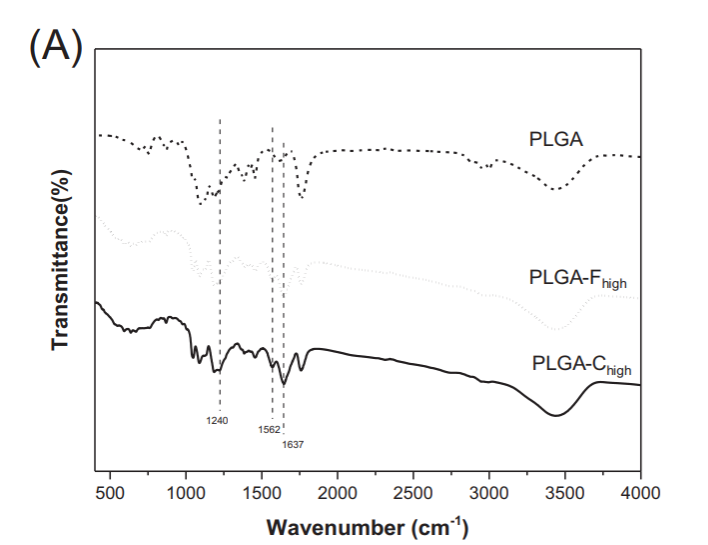
ECM associated proteins (type I collagen and fibronectin) were coupled to PLGA fibres by direct chemical modification with NHS/EDC using two different concentrations of each ECM protein (PLGA-C high: collagen at 100 mg/mL or PLGA-Clow: collagen at 50 mg/mL; PLGA-F high: fibronectin at 50 mg/mL or PLGA-Flow: fibronectin at 25 mg/mL). The scaffolds were checked using SEM to ensure that the porous structure of the scaffolds and fibre morphology were not altered or deformed by the chemisorption process. The scaffolds were then analysed using FTIR in order to confirm the presence of the ECM proteins and BCA assay to measure the levels of protein bound (figure 3).
Figure 3. FTIR analysis of chemisorbed PLGA scaffolds (reproduced from Brown et al's Figure 2)
Cells associated with the scaffolds, coated in two different concentrations of ECM proteins, were then analysed for their liver function and cytochrome P450 expression and compared to cells grown on the unmodified PLGA scaffold. Modification of the scaffold with type I collagen and fibronectin lead to increased urea and albumin production along with higher expression and activity levels of key P450 enzymes. Incorporation of type I collagen onto PLGA scaffolds (PLGA-C high: 100 mg/mL) was the most beneficial, leading to 10-fold greater albumin secretion, 4-fold higher urea synthesis, and elevated transcription of hepatocyte-specific CYP450 genes (CYP3A4, 3.5-fold increase and CYP2C9, 3-fold increase) in primary human hepatocytes compared to the same cells grown within unmodified PLGA scaffolds over two weeks. Brown et al. demonstrate well their ability to produce wet electro-spun PLGA scaffold and discuss the advantages of this over traditional dry electro-spinning. They also report how the modification of the scaffold with ECM proteins can have a beneficial effect on hepatocyte function with high concentrations of type I collagen (100 mg/mL) having the largest impact.
I find this paper extremely thorough, especially when compared to similar papers in the field. They have validated their model well through the use of SEM imaging along with other biochemical techniques and are one of the few papers I have read that quantify live cell numbers within the scaffold after seeding (resazurin assay). My only criticisms of the paper are that the imaging of the cells within the scaffold could be improved. They show small snapshots of the cells clumped in spheres within the scaffold using SEM imaging however, these images could be highly selective and not represent the whole model. They also show H&E staining of the scaffolds but again it is unclear what is actually shown and its relevance. I also feel that an additional comparison between the dry and wet spun scaffolds would be beneficial to the paper. They claim that the new wet electro-spun scaffold is advantageous due to increased pore size that better mimics the acellular liver however, perform no analysis to back up this statement.
-(2).jpg)